At almost exactly the same time that chemists were developing the valence-bond model for coordination complexes, physicists such as Hans Bethe, John Van Vleck, and Leslie Orgel were developing an alternative known as crystal field theory. This theory tried to describe the effect of the electrical field of neighboring ions on the energies of the valence orbitals of an ion in a crystal. Crystal field theory was developed by considering two compounds: manganese(II) oxide, MnO, and copper(I) chloride, CuCl.
Octahedral Crystal Field
Each Mn2+ ion in manganese(II) oxide is surrounded by six O2- ions arranged toward the corners of an octahedron, as shown in the figure below. MnO is therefore a model for an octahedral complex in which a transition-metal ion is coordinated to six ligands.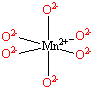
What happens to the energies of the 4s and 4p orbitals on an Mn2+ ion when this ion is buried in an MnO crystal? Repulsion between electrons that might be added to these orbitals and the electrons on the six O2- ions that surround the metal ion in MnO increase the energies of these orbitals. The three 4p orbitals are still degenerate, however. These orbitals still have the same energy because each 4p orbital points toward two O2- ions at the corners of the octahedron.





0 Comments
Thank you !